In HK adult patients resistant to a low-K+ diet, VELTASSA reduced serum K+ within hours1
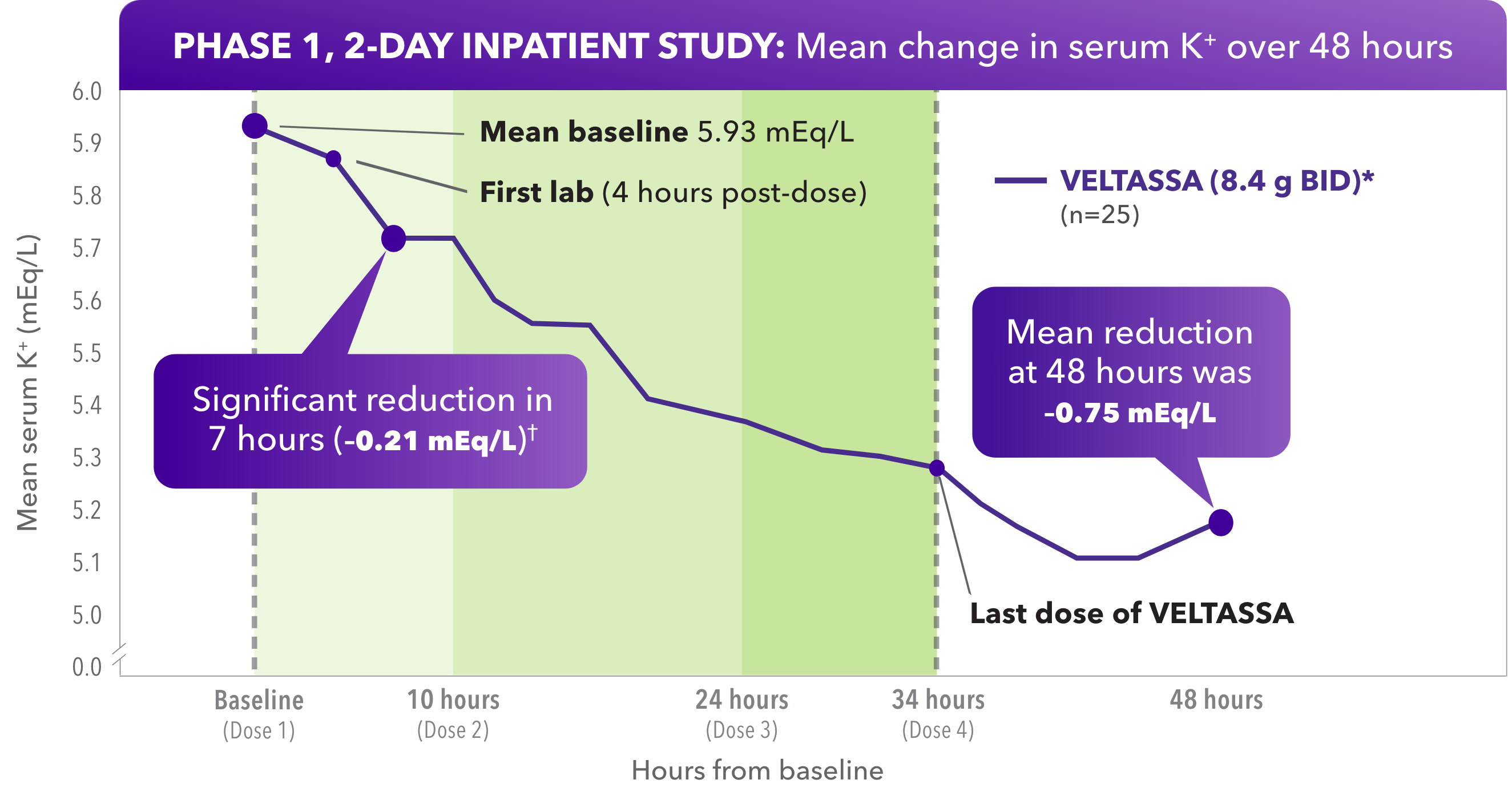
- Significant reduction of serum K+ at hour 7, with reductions starting at hour 4
- K+ diet restricted to 60 mEq/day for patients who were on a potassium-and sodium-restricted diet during the inpatient phase of the study and were counseled to follow a low-potassium diet during the outpatient phase
- 100% of participants were taking 1 or more RAAS inhibitors
- Mean baseline eGFR was 34.8 mL/min per 1.73 m2
Due to its delayed onset of action, VELTASSA should not be used as monotherapy for the emergency treatment of life-threatening hyperkalemia.
Baseline serum K+ = 5.5 to < 6.5 mEq/L.
*BID dosing studied, but not an approved dose.
†P=0.004.
Study design
A phase 1, open-label, single-arm study in 27 patients with CKD and hyperkalemia taking RAAS inhibitors. The study enrolled patients who remained hyperkalemic after a 3-day, controlled diet run-in period. Two of 27 patients had K+ fall below 5.5 mEq/L after the low-K+ diet. The remaining 25 subjects with low-K+ diet–resistant hyperkalemia were dosed with VELTASSA 8.4 g BID for 34 hours. Serum K+ was assessed one hour before treatment: at baseline (hour 0); at 4, 7, and 10 hours post-dose; then every 2 to 4 hours until hour 48; at hour 58; and finally at outpatient follow-up on day 6.
VELTASSA reduced K+ within hours in a real-world study
Next
